Rivers & the Water Cycle Science Lesson
Properties of Water
To learn dive into the river and the water cycle science lesson, let’s start with properties of water. When you hear that NASA’s space probes are looking for “evidence of life” on other planets, do you know what that means? They are looking for evidence of liquid water. Water is fundamental for all life; without it every living thing would die. Water covers about 70% of Earth’s surface and it makes up 65-75% of our bodies (82% of our blood is water). Even though water seems boring – no color, taste, or smell – it has amazing properties that make it necessary for supporting life.
Chemical Composition
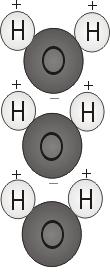
The chemical composition of water is H2O – two hydrogen atoms and one oxygen atom. The way those atoms bond together to form a water molecule is what allows water’s many special properties. The two hydrogen atoms form weak hydrogen bonds with the oxygen; they attach to the top of the molecule rather like Mickey Mouse ears.
Because water molecules are so attracted to one another, they have a high surface tension. The molecules at the surface of the water are attracted to each other and to the molecules below them more than they are attracted to the air. This close attraction forms a type of “skin” on the water, strong enough to support very light objects. Insects that walk on water are taking advantage of this surface tension.
Surface tension is what causes water to clump in drops rather than spreading out in a thin layer. It is also what allows water to move through plant roots and stems and the smallest blood vessels in your body – as one molecule moves up the tree root or through the capillary, it “pulls” the others with it.
This molecular structure gives the water molecule polarity, or a lopsided electrical charge that attracts other atoms. The end of the molecule with the two hydrogen atoms is positively charged. The other end, with the oxygen, is negatively charged. Just like in a magnet, where north poles are attracted to south poles (“opposites attract”), the positive end of the water molecule will connect with the negative end of other molecules.
Water Polarity
Water’s polarity allows it to dissolve other polar substances very easily. When a polar substance is put in water, the positive ends of its molecules are attracted to the negative ends of the water molecules, and vice versa. The attractions cause the molecules of the new substance to be completely surrounded by water molecules. Water dissolves more substances than any other liquid – even the strongest acid! Because of this, it is often called the “universal solvent.” The dissolving power of water is very important for life on Earth. Wherever water goes, it carries dissolved chemicals, minerals, and nutrients that are used to support living things.
Water is the only natural substance that can exist in all three states of matter – solid, liquid, and gas – at the temperatures normally found on Earth. Many other substances have to be super-heated or -cooled to change states. The gaseous state of water is present continually in our atmosphere as water vapor. The liquid state is found everywhere in rivers, lakes, and oceans. The solid state of water, ice, is unique.
Most liquids contract as they are cooled, because the molecules move slower and have less energy to resist intermolecular attraction. When they freeze into solids they form tightly-packed crystals that are much denser than the liquid was originally. Water doesn’t act this way. When it freezes, it expands: the molecules line up to form a very “open” crystalline structure that is less dense than liquid water. This is why ice floats. And it’s a good thing it does! If water acted like most other liquids, lakes and rivers would freeze solid and all life in them would die.
The Water Cycle
Scientists estimate that there is about the same amount of water on Earth today as when it was formed. This water is continually being recycled between the earth, the atmosphere, rivers, lakes, oceans, and living things in a complex process called the water cycle (or hydrologic cycle). Water enters the atmosphere as vapor (its gas form) in two primary ways:
Evaporation
Evaporation accounts for almost 90% of the water vapor in the atmosphere. When water molecules are warmed up – usually by heat energy from the sun – they receive enough energy to change from liquid to gas. Wind increases the rate of evaporation, because it blows wet air away from the surface of the water and replaces it with dry air which can soak up water vapor faster. The majority of water vapor in the atmosphere is evaporated from the oceans.
Transpiration
Transpiration occurs when water vapor is released by animals and humans when they breathe and by plants when they perform photosynthesis.
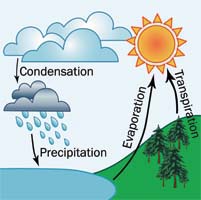 When water evaporates, the vapor is moved up into the atmosphere by rising air currents. The cooler temperatures high in the atmosphere cause the vapor to condense into tiny droplets of water, forming clouds. These clouds are moved around the earth on various air currents, colliding with other clouds on the way. Eventually, water in these clouds falls out of the sky as precipitation – rain, snow, sleet, or hail.
When water evaporates, the vapor is moved up into the atmosphere by rising air currents. The cooler temperatures high in the atmosphere cause the vapor to condense into tiny droplets of water, forming clouds. These clouds are moved around the earth on various air currents, colliding with other clouds on the way. Eventually, water in these clouds falls out of the sky as precipitation – rain, snow, sleet, or hail.
Precipitation
When precipitation falls on land, some of it seeps into the ground and becomes part of the ground water, which feeds wells, springs, lakes, and rivers. Much of the precipitation flows over the ground as surface runoff and joins rivers flowing back to the ocean. Some of it is soaked up by plants and drunk by animals or humans and then put back in the atmosphere through transpiration.
This cycle occurs continually, transporting water from one side of the world to the other. The water you drank today could have fallen as rain on China last year!
Your younger kids might find the full-size PDF version of the water cycle chart helpful.
Rivers
Rivers are important players in the water cycle. They collect run-off from precipitation and move it back toward the oceans. Rivers are also extremely important to our society, providing us with drinking water and irrigation water, helping produce electricity, and allowing us to transport material and food by water. These are some of the reasons that most major cities in the world were founded next to a major river – Rome is on the Tiber, Paris on the Seine, London on the Thames, Cairo on the Nile, and New York City on the Hudson.
Did you know that you are living in a watershed? No, that’s not a type of building! A watershed is an area of land that has a related set of streams and rivers. All the water that falls on that land will eventually flow to the same place – a larger river, lake, or ocean. Some watersheds are small, and some are very large. The watershed of the mighty Mississippi, which drains into the Gulf of Mexico, covers about 40% of the lower 48 states in the US!
What happens in a particular watershed will affect all the water flowing through the outflow point. (The outflow point is the place where all the watershed’s water flows into a larger body of water.) In particular, a river’s streamflow (the amount of water flowing in it) changes constantly because of what happens in its watershed. When it rains in one part of the watershed, rivers and streams will rise in lower parts, sometimes hundreds of miles from where it rained. This could cause flooding even in a place where it hasn’t been raining!
Rivers generally run their course in three stages:
- The upper stage contains the river’s source, which could be a spring, a lake, or a glacier. Many rivers have their source in the mountains, where run-off from melting ice and snow feeds them. In the upper stage, the river bed is often at a steep angle, and the water flows through a V-shaped valley.
- In the middle stage, the river starts flowing through a wider valley. Its bed is more smooth, so the water flows faster. The river begins to meander, flowing in curves from side to side.
- By the lower stage, the river is quite large, since it has been joined by smaller rivers and streams called tributaries. At this stage it usually flows into a sea or ocean.
As the river flows into the sea it begins to deposit sediment that it has been carrying. Often this sediment builds up faster than the sea currents can wash it away, and it forms islands that force the river to split up into many channels. This flat area of islands and channels is called a delta. Deltas contain very rich soil that is ideal for farming. In many countries, such as Bangladesh, people live in the delta and farm there, even though the threat of flooding is always present.
Have you ever wondered why dams are sometimes built across rivers? Dams help control river flow and regulate flooding, but they also use the river to produce hydroelectric power. When a dam is built, water from the river is backed up to form a reservoir.
This water is allowed to flow through the dam to join the river below, and as it does so, its force is used to turn a propeller-like piece of equipment called a turbine. The turbine turns a metal shaft in an electric generator. The electricity that is produced is carried away from the river in power lines. About ten percent of electricity in the U.S. is produced by hydroelectric dams.
Rivers of the World
Rivers assist the agriculture and economy of countries all over the world. Here’s a quick tour of one of each continent’s major rivers.
Let’s start with Africa, because the longest river in the world flows over this continent – the Nile, which is 4,145 miles long. The Nile is usually associated with Egypt, where it forms the great Nile delta and flows into the Mediterranean Sea. Though we tend to think of it as Egypt’s river, the Nile actually flows through 10 African countries: Egypt, Sudan, Uganda, Ethiopia, Eritrea, the Democratic Republic of Congo, Kenya, Tanzania, Rwanda, and Burundi.
The Nile has two main branches: the White Nile, which starts at Lake Victoria in Uganda, and the Blue Nile, which starts at Lake Tana in Ethiopia. These two branches join in Khartoum, Sudan. The Nile is best known for its yearly flood cycle that supported Egyptian life in ancient times. Every year the flooding deposited fertile soil on the farmland. The level of flooding was one of the most important factors in ancient Egyptian economy.
The second longest river in the world is found in South America. The Amazon is 4,000 miles long, and has a huge system of tributaries. Though shorter than the Nile, the Amazon carries much more water: more than the Nile, the Yangtze, and the Mississippi combined! In fact, one-fifth of the water of all the world’s rivers flows through the Amazon.
In places the the river is six miles wide. The Amazon pumps so much fresh water into the Atlantic ocean that the ocean water is drinkable beyond sight of the coastline. The river flows through the Amazon rainforest, and contributes greatly to the wet conditions that helps vegetation thrive, especially during the height of the rainy season when flooding can make the river up to 25 miles wide. The Amazon’s source is in Peru, and it flows through Brazil to empty into the Atlantic Ocean.
Asia claims the third-longest river in the world: the Yangtze, which flows 3,964 miles through China. The Chinese name for the river is Chang-jiang, which means “Long River.” The Yangtze flows by Shanghai into the East China Sea. Ocean-going ships can sail up the river for almost 1,000 miles. The Yangtze is known for severe flooding; especially disastrous were floods in the first half the of the 20th century, killing over 400,000 people. The Three Gorges Dam was built in June 2003 to help with flooding control and to generate hydroelectric power.
In North America, the Missouri river flows into the Mississippi river to form the Mississippi-Missouri river system, the fourth-longest river in the world. Flowing for approximately 3,895 miles from Montana to Louisiana, the Mississippi-Missouri drains half of the water in the lower 48 states.
The Mississippi formed the western boundary of the United States until the Louisiana Purchase in 1803. When Lewis and Clark were sent to explore this new purchase, they traveled along the length of the Missouri on their trek to the Pacific Ocean.
The Murray-Darling river system flows across the continent of Australia. It is the world’s sixteenth-longest river, flowing for 2,310 miles before emptying into the Southern Ocean. The river has a high salt content, which restricts its use to irrigation and the production of hydroelectric power.
One of Europe’s most important rivers is the Danube, which flows for 1,776 miles from its source in the Black Forest (Germany) to its mouth in the Black Sea (Romania). The river flows through or forms part of the border of 10 countries: Germany, Austria, Slovakia, Hungary, Croatia, Serbia, Bulgaria, Romania, Moldova, and Ukraine.
Half of these countries produce a significant amount of their energy from hydroelectric dams on the Danube. The Danube is known to history as one of the long-standing frontiers of the Roman Empire.
Rivers & the Water Cycle Science Projects
Freezing Point
Have you ever wondered why rivers and lakes freeze in the winter, but oceans do not? In this experiment we will see if it is the presence of salt in the ocean that makes it less likely to freeze. You will need a gallon freezer bag, a quart freezer bag, crushed ice, salt, and a thermometer. You may also want some gloves so your hands don’t get too cold!
Fill the gallon freezer bag half full with crushed ice. Add one cup of salt and seal the bag. Put on some gloves and knead the ice and salt until the ice has completely melted. Use the thermometer to record the temperature of the saltwater mixture. Even though the ice has melted, the temperature should be less than 32° F (0° C). Put about an ounce of water in the quart freezer bag. Seal the bag and put in the saltwater mixture in the larger bag. Leave it there until it freezes.
How did the water freeze when surrounded only by saltwater? The salt broke apart the bonds between the water molecules in the ice, causing it to melt, but the temperature remained below the freezing point for pure water. Salt (and other substances dissolved in water) will always lower the freezing point. This is why water in the ocean rarely freezes.
Read the complete water experiments article, with directions for science projects about surface tension and saltwater purification..





