Electricity as we typically think of it, in electronic gadgets, ovens, and lights comes from electromagnetic force. As the name suggests, this force is also what causes a compass needle to point north, or two magnets to stick together. James Clerk Maxwell, a Scottish physicist in the mid-1800s, showed mathematically that electricity and magnetism are actually just two sides of the same force.
One of the keys to understanding electromagnetic force is to know about positive and negative charges of atomic particles. Atoms, the ‘building blocks’ of matter, are made up of tiny particles called neutrons, protons, and electrons. Electrons, the smallest particles, orbit a nucleus formed by denser protons and neutrons, and have a negative charge. Normally an atom has the same number of electrons and protons. The positively-charged protons balance out the electron’s negative charge to make the atom neutral. (Neutrons are already neutral – they have no charge.) If an atom loses an electron to another atom, though, it becomes positively charged because there are more protons than electrons. On the other hand, when an atom gains an extra electron, it becomes negatively charged because there are more electrons than protons. An electromagnetic force results from these different charges being around each other.
Chances are, you’ve heard the saying, ‘Opposites attract.’ This applies to electromagnetism as well. One of the laws of electromagnetism is that like (similar) charges repel and opposite charges attract. What actually happens is that photons, small bits of electromagnetic energy or light, are exchanged between positively- and negatively-charged particles. The more charge something has, the more it attracts or repels. Also, the closer two charges are to each other, the greater the attraction or repulsion between them.
You can demonstrate how charges attract and repel with the following experiment. Hang two inflated balloons from a doorframe or ceiling so that they are just touching. Take a sweater or wool sock and rub the sides of both balloons to negatively charge them – the balloons will pick up extra electrons from the sweater. What happens? The balloons will repel each other – the two negative charges push each other away. What do you think will happen if you stick your hand, which is positively charged, in between the two balloons? Because the balloons have a negative charge, they will be attracted and move toward the positive charge of your hand.
The Law of Change Conservation states that the net amount of electric charge is constant, so electrons can only move from one place to another – they don’t disappear. This means that if one thing gains electrons and becomes negatively charged, something else has lost electrons and become positively charged. In your experiment, the balloons picked up the extra electrons and became negatively charged, while the wool lost electrons.
Static electricity is something you’ve probably experienced: it makes your hair stand up during a pillow fight or shocks your fingers when you touch a cold door handle. Did you know that lightning is a really intense result of static electricity? Static (unmoving) electricity occurs when insulating materials (ones that electric current can’t flow through, such as plastic) get negatively or positively charged. Since the current caused by this force can’t flow through the insulator, the static electricity just sort of sticks to the surface and builds up until it can exchange photons with an opposite charge.
Since lightning is static electricity, it’s not surprising that two results of static electricity are sparks and a crackling sound. You can observe this in a dark bathroom with a rubber or glass friction rod (a comb or a glass rod could also work) and a friction pad or piece of fur or wool. Rub the friction rod against the pad for 15-20 seconds and then touch the rod to the sink faucet. You should hear a crackling sound and maybe even see a small blue spark as extra electrons flow from the rod to the positively-charged metal faucet. Can you think of other ways to get a static electric spark or sound? Are there some materials that work better than others (e.g., a cotton sweater vs. wool, a plastic spatula vs. rubber comb, etc.)? Do more experiments to test your hypotheses!
Science Lesson: Find Out about Batteries & Electromagnets
Batteries were invented in the late 1700s, before much experimentation had been done with electricity. An Italian scientist named Luigi Galvani discovered galvanism (direct electrical current produced by chemical reactions) when he stuck a copper hook through a dead frog and then touched the frog with iron. The frog’s legs jerked every few minutes as if it were alive. Galvani thought this was because the frog had some sort of ‘electrical fluid’ in it. Because of this theory, some people tried to bring human bodies back from the dead by sending electric current through them. This was the ‘scientific’ background for Mary Shelley’s classic, Frankenstein.
A few years later, another Italian, Alessandro Volta, figured out that the electricity in Galvani’s experiment came from a chemical reaction with copper, iron, and body fluids and not from some electric property in the frog. Volta experimented with putting two different metals on his tongue (the fact that it hurt meant electricity must be flowing – his tongue had been shocked) and then made a battery cell by placing wet pieces of paper between two different metals.
A cell, like Volta made, is the ‘powerhouse’ of batteries. It changes chemical energy into electric energy. A simple cell consists of two metal electrodes with different charges (positive and negative) in a liquid or paste electrolyte solution that allows transfer of electrons between the electrodes. Voltaic cells, named after Volta, use two different electrodes, such as zinc and copper or zinc and carbon, suspended in a liquid electrolyte like sulfuric acid or vinegar. Car batteries are an example of voltaic cells.
You can make a simple battery with some pennies and circles of aluminum foil and wet paper towels (soaked in a salt water solution – try one teaspoon salt to 6 oz. tap water) that are the same size as the pennies. Make your battery by stacking 12 ‘cells’ against each other, each cell made up of one penny, one wet paper towel circle, and one foil circle in that order. Wrap a 8-10′ piece of insulated wire with stripped ends around the battery once and twist the ends together against the battery so that the wire holds the cells together. Next, touch the bare ends of the wire to each end of the battery. If you’re in a dark room, you might see a spark as your battery produces an electric current. Another way to test the battery is with a voltage meter or multimeter. Note that U.S. pennies made before 1982 are 95% copper, but newer pennies only have a 2.5% copper coating. For further experimentation, compare the electric current when you make a battery using only older pennies and one using only newer pennies. You could also experiment with a stronger salt water solution or plain tap water.
Batteries produce electric current that flows in a single direction. This is known as DC or direct current. It’s great for portable CD players or flashlights, but it’s not really all that efficient. Due to resistance, some of the electricity gets wasted. Resistance is the slowing down of electrical current through a substance and turning some of that electric energy to heat or light. Copper has low resistance, which is why it is commonly used to make wire. Plastic, on the other hand, has high resistance. This makes it a good coating to keep electricity from escaping a conducting wire.
 In a circuit, the electrons flow from the negative to positive battery terminals. However, thanks to Ben Franklin’s early theories about electricity, scientists were under the impression that current must flow from + to -, and this way of illustrating circuits stuck. As a result, it is standard to talk of current flowing from + to -.
In a circuit, the electrons flow from the negative to positive battery terminals. However, thanks to Ben Franklin’s early theories about electricity, scientists were under the impression that current must flow from + to -, and this way of illustrating circuits stuck. As a result, it is standard to talk of current flowing from + to -.
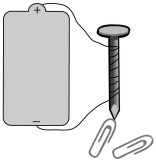
Electromagnets are temporary magnets that only work as a magnet when electrical current is flowing through them. With some insulated wire and a large nail, you can make your own electromagnet. Loosely wrap a long piece of insulated wire around the nail. Connect the ends to a battery and see what happens.
What does the magnet science tell you?
Does your electromagnet attract any metal objects, such as paperclips? What happens if you set a magnetic compass close to the solenoid? Experiment with coiling the wire more times around the nail; does that strengthen the magnetic field so that the electromagnet attracts heavier objects? If you have iron filings, you can test the magnetic field produced by the electromagnet. Sprinkle the iron filings on a paper plate, then hold the electromagnet directly underneath. The iron filings will arrange themselves in the pattern of the magnetic field. Electromagnets are used in electric bells, magnetic levitation (maglev) trains, and much more.
Noteworthy Scientist: Michael Faraday (1791-1867)
There were many great scientists in the Victorian age (c. 1830-1900) who made advances in chemistry, physics, and medicine. Michael Faraday was one of these scientists. He was born near London in 1791. Faraday, the son of a blacksmith, went to day school and then, at age 13 or 14, was apprenticed to a bookbinder. During this time he began to develop an interest in science and performed some chemistry experiments. He joined the City Philosophical Society to discuss scientific findings and gave his first scientific lectures there.
In 1812, near the end of his apprenticeship, Faraday was given tickets to attend some lectures by Humphry Davy, a famous chemist. Faraday made a book of his notes from the lectures, which he presented to Davy and then asked him for a job. Davy turned him down, but a year later there was an opening for Chemical Assistant at the Royal Institution, and Davy appointed him. Later in the year, Faraday went along as Davy’s assistant on an 18-month scientific tour of Europe. Afterward he continued to work for the Royal Institution and do experiments in the lab there. He was promoted in 1821 and got married the same year.
Although Faraday performed notable chemistry experiments, among them liquefying chlorine to prove that a gas could be turned into a different state of matter, he is best known for his work in electromagnetism. In 1821 he discovered electromagnetic rotation, the principle behind electric motors. Ten years later, he discovered electromagnetic induction which was the force behind the electric generator that he invented. After that, he and a classicist developed words we still use to describe electrical phenomena: ‘electrode,’ ‘electrolyte,’ and ‘ion’ to name a few. In 1845 Faraday investigated the relationship between light and magnetism, doing an experiment which showed that light could be affected by magnetic force. This was later called the Faraday Effect. His theories were later put in mathematical form by James Clerk Maxwell, making them a foundational physics concept.
Later in life, Faraday worked to make lighthouses more efficient (he invented a special chimney for oil lamps), taught chemistry at the Royal Military Academy, and even was an expert witness in a trial. He was a member of the Sandemanian church, a denomination which believed in a strict literal interpretation of the Bible, and gave a lecture at the Royal Institution against spiritualism, which was first becoming popular then. Although he had worked closely with the Royal Institution for most of his life, in 1864 Faraday turned down an offer to be its president. In addition to being known for his humility, Faraday gave to many charitable causes and started yearly ‘Christmas lectures’ about science for children. You can read some of Faraday’s 1827 Christmas lectures here.
Science Links
Find out about static electricity and Van de Graaff generators at this Theater of Electricity museum site.
Get interesting experiment ideas at Energizer’s Science Projects site.





