







category
Kitchen Science Kits

Combining flavor, fun & chemistry, this spherification kit is a great & tasty intro to food science! Use food-grade versions of three chemicals used in molecular gastronomy recipes to create colorful, edible spheres!
Ideal for Grades 6+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

This orange juice titration kit is perfect for an aspiring chemist interested in trying his or her hand at real-world chemistry. Learn all about titration as you explore vitamin C levels in fruits and vegetables!
Ideal for Grades 9+
Out of Stock, Expected to Ship: 04/27/2024
Need It Fast? See Delivery Options In Cart.

Use this fun chromatography kit to discover the hidden colors in dyes all around you!
Ideal for Grades 2+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.
Item ships to the United States only.

If you are what you eat, what makes up your food? Experiment and learn about the connections between chemistry & nutrition for a meaningful STEM activity.
Ages 11+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.
Item ships to the United States only.

There's science in your ice cream! Explore the chemistry behind getting your homemade ice cream to freeze.
Ideal for Grades 6+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Explore Candy Chemistry with this kitchen science kit from Thames & Kosmos! This creative science kit provides an excellent way for kids to learn about the science behind a number of delicious candy flavors and textures.
Ages 10+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Test fruits, veggies & drinks for vitamin C while learning about the chemical components of different foods! This hands-on kit provides all the necessary chemicals & lab equipment to perform 6 different vitamin C experiments.
Ages 11+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Grow your own rock candy crystals with this unique Crystal Growing Experiment Kit! Kids will love this delicious sugar crystal science experiment; edible science is a perfect way to engage curious learners!
Ages 5+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Unicorns, clouds & rainbows... oh my! With the Thames and Kosmos Rainbow Gummy Candy Lab, kids learn about the scientific properties of natural polymers while they make their own delicious, fun gummy shapes.
Ages 6-12
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Make cheese at home with this mozzarella and ricotta cheese making kit! Fun for the whole family.
Ages 10+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Use the Bubble Gum Chemistry Kit to make chewy long molecules, spheres of dubious strength, popping sounds, and viscoelastic bubbles. Delicious polymers & loudish sounds!
Ages 8+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.
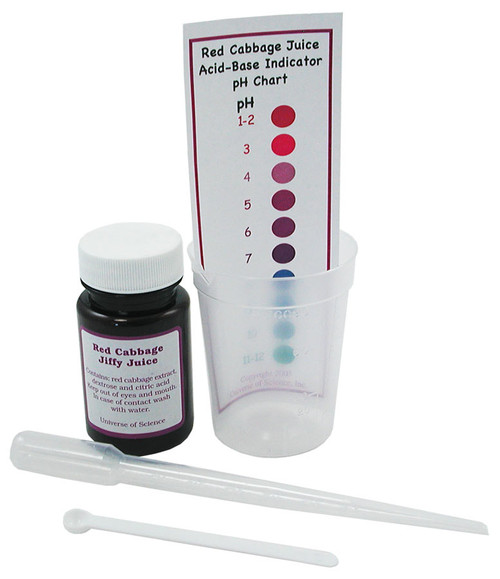
The indicator inside this Red Cabbage Jiffy Juice Kit contains concentrated red cabbage juice to detect pH levels. Use the pH indicator to test things around your house, like laundry detergent, soda pop, and more!
Ages 9+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Transform your kitchen into a science lab! Built around common kitchen items, this kit uses fun kitchen science experiments to give kids entirely new ways to look at food. Doing science has never been more delicious!
Ages 12+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.
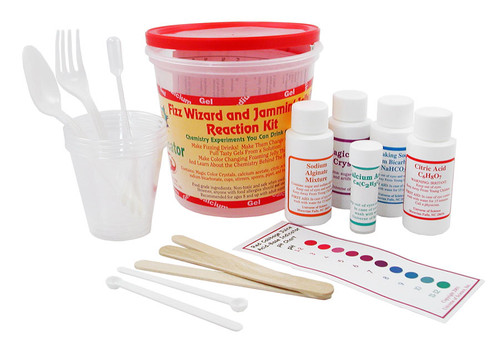
This fun kit introduces basic chemistry concepts through making incredible and tasty concoctions like soda pop and pudding.
Ages 8+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

Make your own shampoos and test how well they remove greasy build-up with this fun and educational Science Buddies kit. From jojoba oil to graduated cylinder and 100% wool to simulate hair, this kit contains everything you need.
Ideal for Grades 6+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.

You've seen the MENTOS geyser eruption online. Now experience the soda geyser in your own backyard with the Geyser Tube from Steve Spangler and Be Amazing Toys.
Ages 6+
In Stock & Ready to Ship
Need It Fast? See Delivery Options In Cart.
Turn your kitchen or classroom into a science lab with kitchen science kits.
Students will mix up tasty treats and experiment with food science. Finally, kids have a reason to play with their food!
Since everybody eats every day, kitchen science kits for kids are an easy way to highlight how science is relevant in normal life. And since many students love learning activities that involve food, these kits please teachers and kids alike. Plus, many of them include prompts for science fair projects.
Kitchen science kits for kids teach chemistry concepts through memorable—and edible—projects. Students can make and eat their own chocolate, cheese, ice cream, and sugar crystals.
Discover the nutrients within common foods using a Chemistry of Food Experiment Kit. Explore chromatography with candy, test foods for vitamin C content, and more!
Kitchen science kits include the science supplies, chemicals, and necessary non-perishable food items. Perishable food items, common household supplies, and optional items, are typically not included.
We get it. Science can be messy. But Home Science Tools' products and service can handle it.
Our products are durable, reliable, and affordable to take you from the field to the lab to the kitchen. They won't let you down, no matter what they're up against. Whether it's (over)eager young scientists year after year, or rigorous requirements that come once-in-a lifetime.
And if your science inquiry doesn't go as expected, you can expect our customer service team to help. Count on friendly voices at the other end of the phone and expert advice in your inbox. They're not happy until you are.
Bottom line? We guarantee our products and service won't mess up your science study—no matter how messy it gets.
Questions? Get in touch with our Customer Service team.